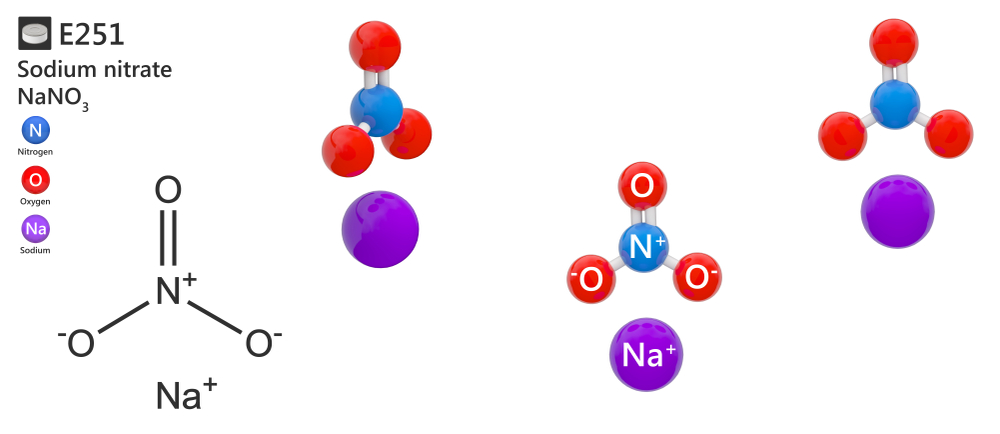
Sodium nitrate is an inorganic substance, the sodium salt of nitric acid (V). The sum formula is NaNaO3.
The appearance of sodium nitrate
Sodium (V) nitrate is in the form of white fine crystals, has no odour and is characterized by a salty-bitter taste. It dissolves well in water. Chile saltpetre is hygroscopic. That means that it can absorb water from the environment. It shows oxidizing properties, is itself non-flammable but increases the combustion of other substances. On an industrial scale, sodium nitrate is obtained in the reaction of nitric acid (V) with sodium carbonate or sodium hydroxide.
Sodium nitrate - applications
Sodium nitrate has a wide range of applications. Among the primary ones is its use as a food additive designated with the symbol E251. Sodium nitrate in food is a preservative which mainly prevents the growth of botulism bacteria Clostridium botulinum. Nitrates in meat additionally prevent the colour change and are responsible for their characteristic pink colour. Sodium nitrate in agriculture is a very important product. It is a component of fertilizers enriching the soil with nitrogen necessary for plant growth. In addition, sodium nitrate is used:
- in adhesives and sealants
- in the manufacture of explosives, including nitroglycerine
- in solvents
- in the production of paints and varnishes
- as an oxidizing agent in pyrotechnics
- in the glass and ceramics industry
- as a substrate for the production of potassium nitrate
Sodium nitrate in food – where we can find E251?
Sodium nitrate is found in almost all vegetables. Plants absorb it up from the soil and use it for growth. In areas where significant amounts of nitrogen fertilizer are used, the amount of nitrate in vegetables is higher. Most sodium nitrate is found in root vegetables, such as carrots, parsley, and beets. According to scientific reports, about 80 per cent of nitrate consumed in the diet comes from vegetables. The remaining sources are drinking water and processed food such as sausages, canned meat and fish, and some cheeses.
Safety of sodium nitrate
Sodium nitrate itself is not harmful to health, but toxicity is shown by other nitrogen compounds that can be formed from it in the digestive tract. When exposed to temperature, as well as the low, acidic pH in the stomach, about 25 per cent of nitrates are converted to nitrites, and these are then converted to nitrosamines to some degree.
The scientific community treats nitrosamines as potentially carcinogenic substances for humans. It is therefore not possible to talk about the carcinogenicity of sodium nitrate, but about the carcinogenicity of nitrosamines, which can be formed from sodium nitrate at very high intakes.
The acceptable daily intake (ADI) of E251 is 3.7 mg per kilogram of bodyweight. The average intake of nitrates in Europe does not exceed the ADI.
A real danger to infants and young children is water containing high levels of nitrate. After conversion to nitrite, methemoglobinemia can occur, a condition in which haemoglobin loses its ability to bind oxygen and hypoxia of the nervous system and brain occurs.
Should you eat vegetables rich in sodium nitrate?
There is no reason to give up vegetables rich in sodium nitrate for fear of health consequences. It is important to remember that only a portion of sodium nitrate is converted to nitrite, plus vegetables are very rich in antioxidants and vitamins that neutralize the harmful effects of nitrite. The benefits of eating plenty of vegetables significantly outweigh the possible risks associated with consuming sodium nitrate.
Remember that the DASH diet, which is considered the healthiest diet of all based on many years of in-depth studies, is based on vegetables, and during its use, you can exceed the acceptable daily intake of sodium nitrate by up to 5 times.