Nitrates are found mainly in vegetables as a result of the use of fertilizers, and nitrites - in meat preparations, where they are used as a preservative against botulism. Nitrogen compounds can also be supplied with drinking water. Excessive amounts of nitrogen compounds in food is dangerous to health and can cause, among others cyanosis, anaemia, bowel disorder and cancer. In this article, we will suggest how to avoid excessive consumption of nitrates
Nitrates and nitrites - toxicity
Nitrates and nitrites are nitric acid derivatives. Nitrates come from nitric acid (V) HNO3, and nitrites come from nitric acid (III) HNO2. The number of oxygen atoms with which nitrogen is bound in a chemical substance affects its properties.
Nitrates are marked as generally safe for humans, and nitrites become toxic at too high doses. Nitrates are present in food mainly of plant origin due to the use of artificial fertilizers. As a result of fertilization, they also reach surface waters and can be found in tap water.
They can also be found in feed and water fed to farm animals. Nitrates and nitrites are used in the production of various types of meat preparations and cheeses. Their role is primarily being preservative extending the time of which food can be safely consumed.
Nitrogen compounds inhibit the growth of pathogenic microorganisms (mainly Clostridium botulinum, which produce a strong toxin called botulinum toxin), act as antioxidants and improve the sensory qualities of the finished product, e.g. maintain its desired colour.
In the dairy industry, they are used in the production of ripening cheese, in which they prevent re-fermentation of lactic acid and so-called “flatulence” of cheese.
Nitrogen compounds can be toxic to humans, which is why the European Commission Regulation of 2006 strictly sets the maximum content of nitrates and nitrites in food products. According to the World Health Organization, the acceptable daily intake (ADI) for nitrates is 5 mg/kg of body weight, and for nitrites - 0.1 mg/kg of body weight.
This is due to the fact that nitrates themselves are not toxic to humans, however, in the acidic stomach environment at pH<4 (remember that our stomach is filled with hydrochloride acid with pH close to 1!) and also under the influence of bacteria present in the digestive tract, they are transformed into nitrites, which are excessively hazardous to health.
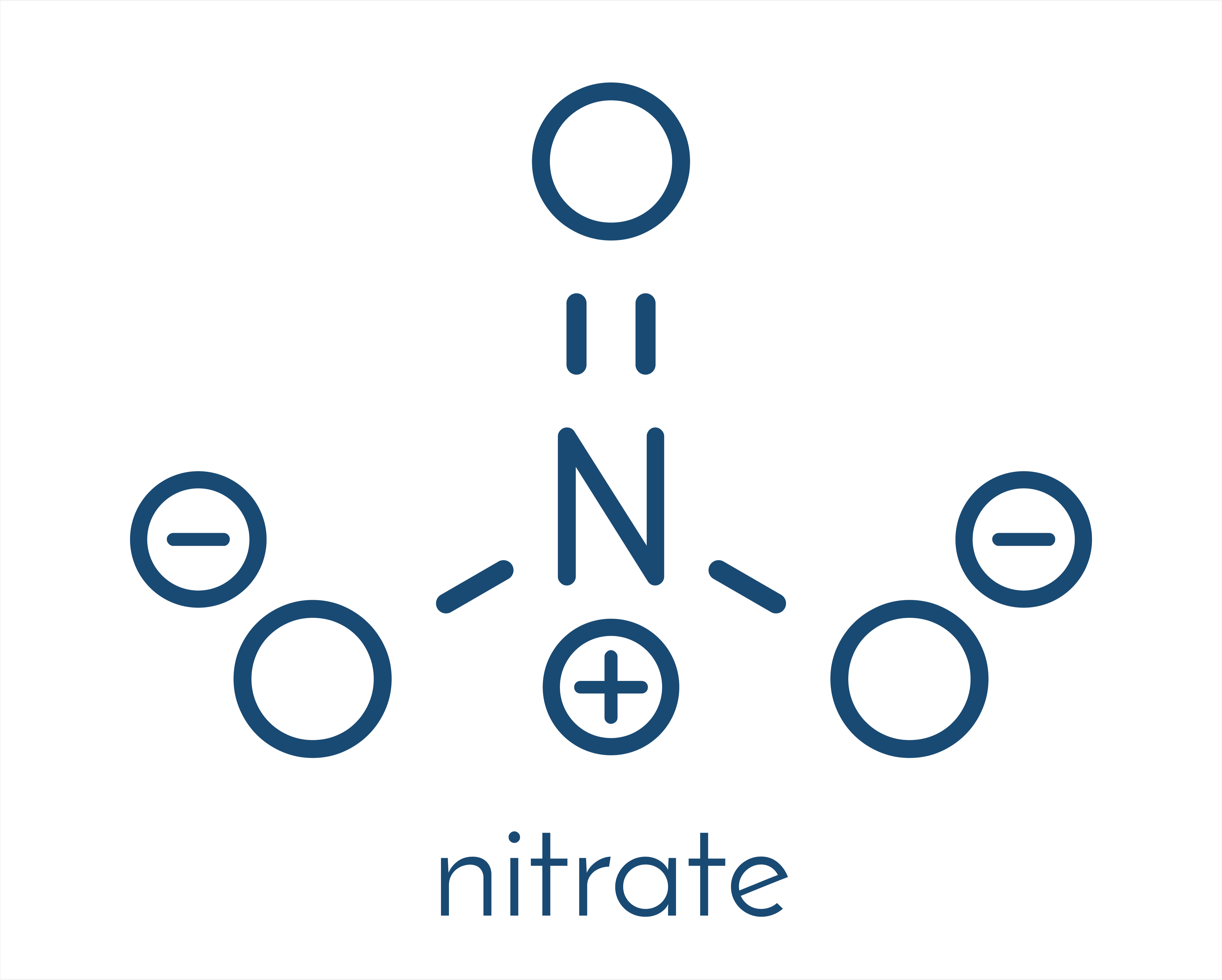
Nitrates and nitrites - sources in food
Based on the estimated assessment of the intake of nitrates and nitrites in food in 1996-2005 it was found that the main source of nitrates in the diet are vegetables and provide on average 89% of these compounds, and nitrites are mainly supplied (in 69%) from meat and meat preparations.
It is important that the average diet does not exceed the permissible daily intake of nitrogen compounds. The average nitrate uptake is 132-190 mg NaNO3/person/day, i.e. 56.8% ADI, and nitrite - 3-3.5 mg NaNO2/person/day, which is 58% ADI.
Fresh vegetables contain nitrates and nitrites only in small quantities. However, their concentration increases during storage. A large amount of nitrates in vegetables results from the excessive use of fertilizers in industry.
The nitrate content also depends on the plant species and environmental conditions. Vegetables are divided into categories due to the tendency to store nitrates
The largest amount of nitrates is found in root vegetables and tubers and in vegetables intended for an early harvest. These compounds can accumulate in different parts of plants.
Generally, it can be assumed that the more thick and hard the part of the plant, the more nitrates it contains. For example, the cucumber has the most of it in the skin, in broccoli and cauliflower - in the stalk, in cabbage - in the depths and the outermost leaves, and in carrots - at the end of the root.
During cooking, the nitrate content of vegetables is reduced by about 50%. The concentration of nitrates in juices is similar to their quantity in fresh vegetables.
Nitrites in cooked meats and cheese
Nitrites are commonly used as a preservative in meat preparations, hence 69% of these compounds in the diet come from sausages and sausages.
Milk and dairy products only provide 3.2% of the nitrate in the diet. Due to the growing awareness of consumers and aversion to nitrite, manufacturers often put the information E250 on the product labels - a preservative instead of the chemical name sodium nitrite.
However, it must be remembered that certain amounts of nitrogen compounds must be present in meat preparations to ensure their microbiological safety. The more meat and fewer additions the sausage contains, the better.
In raw meat, nitrites are virtually absent and provide only about 1% of these compounds in the diet. According to the standards, maximum 150 mg/kg of sodium nitrite can be found in cured meat and other meat preparations, and 100 mg/kg in canned products.

Nitrates and nitrites - are they hazardous to health?
Nitrates are compounds generally safe for humans, but consumed in high concentrations can irritate the small intestinal mucosa and cause malabsorption.
Approx. 25% of nitrates are converted to toxic nitrites, and these may form carcinogenic nitrosamines. Nitrites and nitrosamines have a wide negative impact on health
Nitrogen compounds in food and methemoglobinemia
Nitrites oxidize iron ions contained in haemoglobin, resulting in methaemoglobin. The red blood pigment loses the ability to transport oxygen, which leads to hypoxia of the central nervous system and myocardium.
Symptoms are stronger the more methaemoglobin circulates in the blood and the tissues are more hypoxic. Cyanosis, the so-called blue baby syndrome is especially dangerous in infants and young children who can poison with nitrogen compounds present in water and food.
Nitrates are transformed into toxic nitrites in their bodies much faster. In a healthy body, the concentration of methaemoglobin (MtHb) is not higher than 1-2%.
Nitrogen compounds and cancer
Nitrates and nitrites are precursors of nitro compounds that have confirmed carcinogenic effects and cause fetal malformations by damaging genetic material. In experimental animals, already small doses of nitrosamines (5 mcg/g) cause cancerous changes, and in food products, there can be even over 500 mcg/g of them!
The most dangerous is food, to which nitrites are added as a preservative, i.e. sausages and other meat preparations because nitrosamines are formed from nitrites under the influence of high temperature.
These compounds influence the development of cancer of the oesophagus, stomach, large intestine, pancreas, prostate, ovaries and breast, and in children increases the risk of developing leukaemia.